

SteriKit™

Transport swab system with growth indicator
for accelerated detection of contamination.
The Sterikit™ System
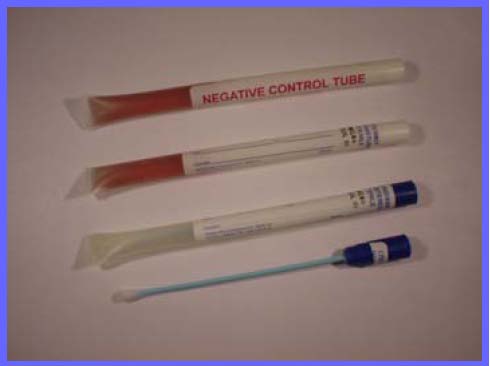
Each test kit consists of two components.
1. Sterikit™ Tube containing BP* specified Tryptone Sot Broth Medium with growth indicator
2. Steriswab™, premoistened swab with blue plastic shaft and rayon bud.
Method of Use
1. Take one sterile Steriswab and one Sterikit™ Tube
2. The Steriswab™ is removed from the tube and is used to sample the test surface.
3. The Sterikit™ Tube can now be removed to the laboratory or testing area, and incubated at 37° C.
Any microbial growth occurring in the tube will cause the indicator to change from dark red to yellow. A Negative control
is supplied in each pack, so that any change can be quickly seen.
Sterikit™ is intended to reveal any microbial contamination , but will not give specific information about the types of
organisms. If required, where a positive result is obtained, further testing can be done on the Steriswab™ using
standard microbiological techniques.
Presentation
Bulk sets of each component are included pack of 50 tests, and are triple wrapped before sterilization by irradiation,
to ensure that not only the swab and medium are sterile, but all components are externally sterile for handling
within a clean or sterile areas. Each pack contains a negative control tube of Sterikit™ medium for reference.
The flexible and convenient method of environmental monitoring and sterility testing for the
pharmaceutical microbiologist.
Sterikit™ combines the direct culture of a contact plate, the reach and surface release pressure of a
swab, and the convenience of a transport tube, all in one self-contained, easy-to-use kit.
Rayon bud
Swabs produced with rayon (cellulose) buds for optimum recovery of all microorganisms
SteriKit™ medium
SteriKit™ medium complies with BP-specification and meets all growth and recovery requirements.
Each batch of SteriKit™ medium is tested for the recovery of BP*-specified organisms, (see product insert.
Anaerobe/Aerobe Recovery
The semi-solid presentation of the growth medium enables the recovery and growth of anaerobic, microaerophilic and
aerobic organisms. Quality Assurance Certificate supplied with each pack.
Stressed Organism Recovery
Optimized semi solid medium formulation and BP*- recommended buffer solution enables swab and medium to
maximize recovery and growth of stressed organism.
Swab shaft allows sampling in less accessible areas.
Unlike contact plates which can only be used against flat surfaces, Steriswabs™ can be used to reach into, or behind
equipment. The swab can be pressed hard against surfaces to release organisms from any possible biofilms.
Biofilm release
Standard contact plates, slides, agar strips etc., are not well suited to releasing microorganisms from the biofilms they
create, a problem which is increasingly recognized. The use of a premoistened swab device allows wetting and
mechanical pressure to be directed at the test area, with enhanced removal and collection of any organisms present.,
and increases the probability of detection.
Larger Test Area
By its nature, the swab technique is not limited to the test area, it may accurately monitor a larger surface area than the
contact plate/agar strip method. SteriKit™ offers more effective and representative sampling over larger test areas.
Visible results -every time
Growth indicator system provides early and visible color indication of the presence or absence of even low level
contamination at test area.
Convenient to use - Easy to read
Visible color indication of contamination reduces reading/ interpretation time from hours to minutes. Hours of
screening is replaced by a studied observation of racks of tubes and comparison with control tubes -completed
and confirmed in moments.
SAVE $ - Compared to standard contact plate and swabbing techniques the SteriKit™ system offers significant value per test.
Triple Wrapped For Maximum Sterility Sterility is guaranteed with triple wrap for confidence in sterile and
clean working areas.
MANUFACTURED TO THE STANDARD OF A STERILE MEDICAL DEVICE
Sterikit™ is manufactured by M W & E, the makers of Transwab®. M W & E. is accredited for the manufacture
of Class IIa sterile medical devices in accordance with the European Medical Devices Directive (93/42/EEC) FDA:
GMP Accredited Manufacturing Site, Owner / Operator No. 8021919
* British Pharmacopoeia
| © Lakewood Biochemical Co., Inc. Dallas, TX 75214 |
Index , Alphabetical by Subject Titles
GENERAL CATALOG SECTIONS
